4 min read
Uses of Metals: Types, Classifications and Applications
Metals are among the most important and widely used materials by humankind.From prehistory to Industry 4.0, they have supported technological...

Metals are among the most important and widely used materials by humankind.
From prehistory to Industry 4.0, they have supported technological development thanks to unique properties such as mechanical strength, conductivity, ductility and durability.
In this guide we will look at:
Metals are chemical elements characterised by:
From an atomic point of view, they tend to lose electrons, forming metallic bonds that explain many of their physical properties.
These characteristics make metals ideal for processes such as CNC milling, CNC turning and precision machining, which are essential in modern industrial manufacturing.
Metals are essential because they:
Without metals, there would be no:
For this reason, choosing the right metal material is one of the most critical aspects in the design of industrial components.
Upload your file and speak with an expert
Metals can be classified in different ways. The two most common classifications are:
Ferrous metals are those that contain iron (Fe) as their main element.
Examples
Features
Main uses
Thanks to alloying and heat treatments, steels are the most widely used metals in the world.

Non-ferrous metals do not contain iron as their main element.
Examples
Features
Main uses

These are the most reactive metals of all. They are found in the first column on the left of the periodic table (excluding hydrogen).
Elements: Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb), Caesium (Cs), Francium (Fr).
Chemical characteristics: They have only one valence electron, which they readily lose to form monovalent cations.
Reactivity: TThey react violently with water, forming hydroxides (alkalis) and releasing hydrogen. They are never found in nature in their pure state, but always as compounds (salts).
Physical properties: They are soft (can be cut with a knife), have low density and low melting points.
They are very reactive, but less so than alkali metals.
Elements: Beryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Ba), Radium (Ra).
Chemical characteristics: They have two valence electrons, which they lose to form divalent cations).
Reactivity: They react with water (often requiring heat) and oxidise easily in air. Their oxides react with water to form alkaline solutions.
Strength: Magnesium and beryllium are exceptions in this group: they are very stiff and lightweight, which is why magnesium is used in racing car alloys and ultra-light laptop frames.
They make up the large central group of the periodic table (Groups 3–12).
Elements: Iron (Fe), Copper (Cu), Gold (Au), Silver (Ag), Titanium (Ti), Zinc (Zn), etc.
Chemical characteristics: Their defining feature is the filling of d orbitals. They can have multiple oxidation states (e.g. iron can be +2 or +3), allowing them to form a wide range of coloured compounds and often act as catalysts.
Physical properties: They are generally hard, with high melting and boiling points, and are excellent conductors of electricity and heat.

They are located to the right of the transition metals, before the metalloid boundary line. They are sometimes called “poor metals”.
Elements: Aluminium (Al), Tin (Sn), Lead (Pb), Gallium (Ga), Indium (In), etc.
Chemical characteristics: They have valence electrons in p orbitals. They are chemically “weaker” than transition metals: softer and with lower melting points.
Reactivity: They tend to form covalent bonds more often than alkali metals and often show amphoteric behaviour (they can react with both acids and bases, like aluminium).
They are positioned in the two rows at the bottom of the periodic table.
Lanthanides (Rare Earths): Similar to lanthanum. They are silvery, highly reactive metals widely used in high-tech applications (e.g. neodymium magnets, displays).
Actinides: Similar to actinium. They are all radioactive. The most well-known are uranium (U) and plutonium (Pu).

In industrial practice, pure metals are rarely used.
Alloys make it possible to:
Examples
The future points towards:
Metals are the foundation of modern industrial civilisation.
Understanding their types and uses is essential to choosing the right material based on:
Do you need to manufacture a metal component?
With Weerg, you can machine aluminium, steel, stainless steel, brass, copper and bronze using 5-axis CNC machining, with instant online quotations and specialised technical support.
Upload your CAD file and get an immediate CNC quote
The properties of metals describe their physical, mechanical, chemical and manufacturing behaviour. The main ones are:
Physical properties:
electrical and thermal conductivity, density, melting point, magnetism.
Mechanical properties:
strength, hardness, ductility, malleability, toughness, elasticity, Young’s modulus.
Chemical properties:
corrosion resistance, chemical reactivity, passivation ability.
Technological properties:
machinability, weldability, castability, heat-treatability and recyclability.
Together, these properties make metals fundamental materials for construction, industry, transport, electronics and energy.
Metals are a class of chemical elements (or alloys) characterised by high electrical and thermal conductivity, mechanical strength, ductility and malleability.
They are generally solid at room temperature, have a shiny appearance and can be easily processed through industrial methods such as casting, rolling and machining.
Metals can be classified according to two main criteria: industrial and chemical.
1) Industrial classification
2) Chemical classification (periodic table)
This dual classification helps in selecting the most suitable metal based on properties, application and manufacturing process.
The most widely used metal in the world is steel, an alloy based on iron and carbon.
It is used in enormous quantities because it combines high mechanical strength, relatively low cost, versatility and ease of processing.
Steel is essential in:
Thanks to the ability to create hundreds of different alloys, steel can be adapted to almost any industrial application.

4 min read
Metals are among the most important and widely used materials by humankind.From prehistory to Industry 4.0, they have supported technological...
2 min read
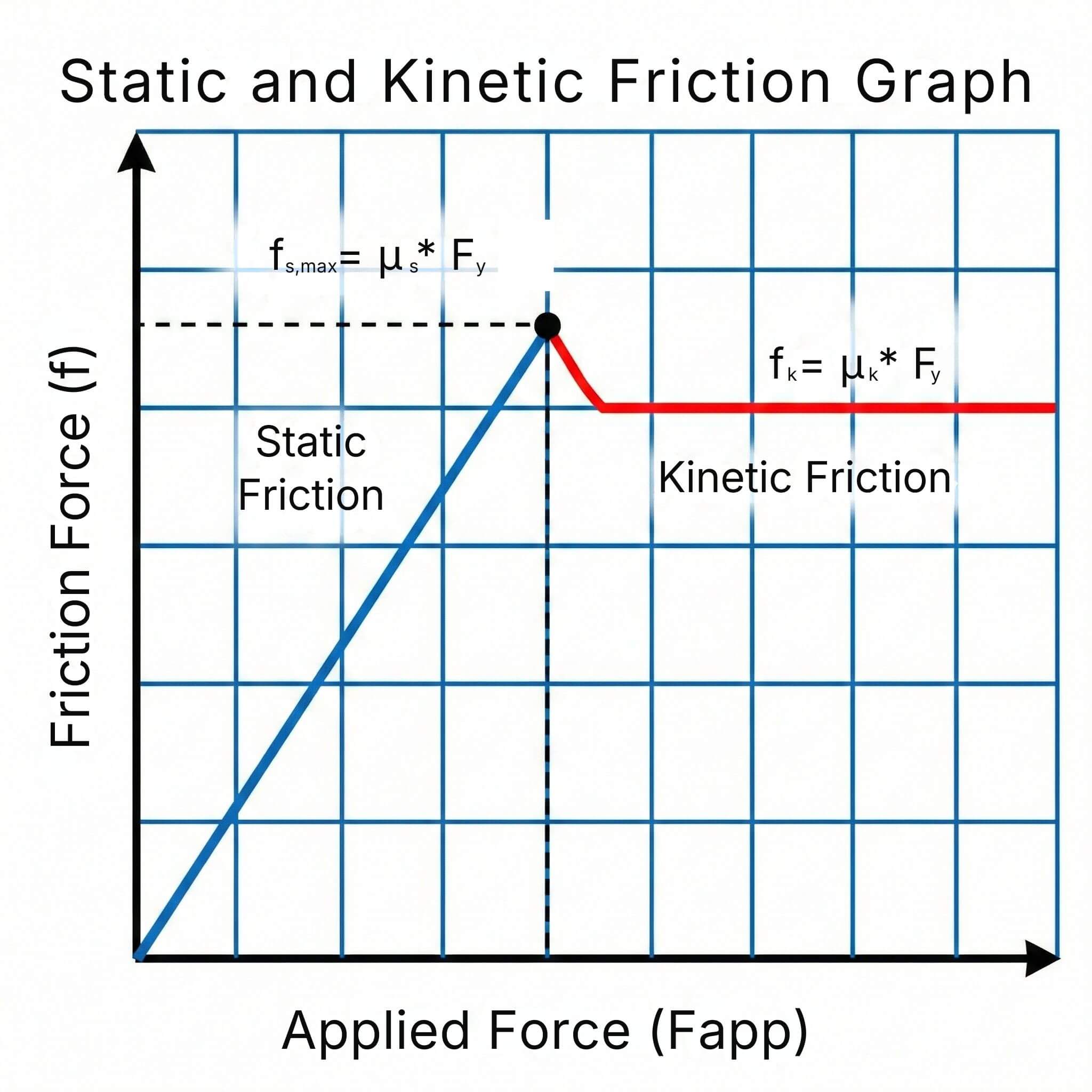
The coefficient of friction (μ) measures the resistance to sliding between two surfaces in contact. It is a key parameter in mechanical design...

3 min read
The CBAM regulation (Carbon Border Adjustment Mechanism) represents one of the key pillars of the European strategy for industrial decarbonisation...