2 min read
Coefficient of Friction: what it is and why it is essential
The coefficient of friction (μ) measures the resistance to sliding between two surfaces in contact. It is a key parameter in mechanical design...
3 min read
![]() Weerg staff
:
Dec 23, 2025
Weerg staff
:
Dec 23, 2025

Galvanisation is one of the most effective and widely used surface treatments for protecting steel and other metals from corrosion, oxidation and wear.
Used for over 150 years in construction, mechanical engineering and the automotive industry, it is now an essential industrial standard for increasing the durability, strength and reliability of metal components.
In this guide you will find:
Galvanisation is a metallurgical process that consists of applying a protective layer of zinc to the surface of steel or another metal.
The name derives from Luigi Galvani, the discoverer of bioelectricity, but modern industrial application is based on the principle of electrolysis.
Zinc acts as a corrosion barrier and also provides electrochemical protection, preventing the underlying steel from rusting.
In practice, galvanisation:
protects the metal from atmospheric agents
prevents rust
increases component lifespan
improves aesthetic appearance
reduces maintenance costs
The principle behind it is galvanic action: zinc, being more reactive, corrodes in place of the steel (sacrificial protection).
The process varies depending on the technique used, but generally includes:
degreasing
removal of oxides and impurities
pickling: the part is immersed in acidic solutions to remove existing oxides (surface rust)
This can be done by immersion, electrodeposition, spraying or thermal diffusion.
The zinc adheres to the surface, forming a uniform protective layer.
Checking adhesion, thickness and coating uniformity.
There are several techniques, each with specific characteristics and applications.
| Type of galvanising | How it works | Typical thickness | Corrosion resistance | Main advantages | Limitations | Ideal applications |
| Hot-dip galvanising | Immersion of the part in molten zinc at ~450°C | 50-150 μm | Very high (decades) | Durable protection, resistance even in marine environments | Possible aesthetic irregularities, not suitable for tight tolerances | Metal fabrication, construction, outdoor structures, guardrails, poles |
| Electroplated galvanising (electrodeposition) | Zinc deposition using electric current | 5-20 μm | Medium | Uniform and highly aesthetic finish, precise thickness controls | Lower resistance than hot-dip galvanising | Small hardware, screws, bolts, precision mechanical parts |
| Spray galvanising (metallisation) | Molten zinc sprayed using thermal guns | 40-80 μm | High | Suitable for very large parts, ideal for repairs | Requires thorough surface preparation | Large structures, local repairs, construction sites |
| Sherardising (thermal diffusion) | Zinc diffusion in rotating drum at 350°C | 20-80 μm | High | Very uniform coating, excellent for threads | Not ideal for large parts | Fasteners, complex components, parts subject to friction |
| Mechanical galvanising | Application of zinc particles through mechanical processes | 8-30 μm | Medium | Excellent for small batch components | Not suitable for aggressive environments | Bolts, small components or heat-sensitive parts |
It is important to distinguish electroplated galvanising (ideal for controlled thicknesses and precision) from hot-dip galvanising, which involves immersion in molten metal.
In precision engineering, the electroplated process is often preferred to avoid altering critical dimensional tolerances.
Immersion in molten zinc produces a thick layer composed of metallurgical alloys that integrate with the steel.
This characteristic gives the coating:
high mechanical strength
excellent corrosion protection
long service life even outdoors or in saline environments
It is therefore a solution aimed at maximum protection rather than aesthetic precision.
In the electroplating process, zinc is deposited by electric current as a thin, uniform film.
The thickness can be precisely controlled, and the resulting finish is much cleaner and more aesthetic than hot-dip galvanising.
Corrosion protection is good but lower, and it is intended mainly for mechanical components or parts with tight tolerances.
Hot-dip → more protective, thicker, more resistant
Electroplated → more precise, more aesthetic, less protective
The two techniques do not compete: they meet different needs.
Galvanisation works best with steel and cast iron, as zinc adheres stably and provides very high corrosion protection.


In summary: galvanisation is ideal for steel, useful in some cases for cast iron and a few other metals, but generally not recommended for non-ferrous alloys.

Automotive: Fasteners, brackets, chassis components exposed to salt and moisture
Electronics: Often used with gold or silver plating not for corrosion protection, but to improve contact conductivity
Construction and metal fabrication: External load-bearing structures
Marine environments: Pontoons, barriers, equipment exposed to salinity.
Galvanisation is a fundamental process for protecting steel from corrosion, improving durability and reducing maintenance costs.
Between hot-dip, electroplated and thermal diffusion zinc coating, each method offers specific advantages for different requirements.
If you need to manufacture metal parts intended for outdoor use, aggressive environments or long service life, galvanisation is one of the most reliable and cost-effective solutions available.
Do you want to manufacture CNC components?
Upload your file now and get an instant quote with professional finishes
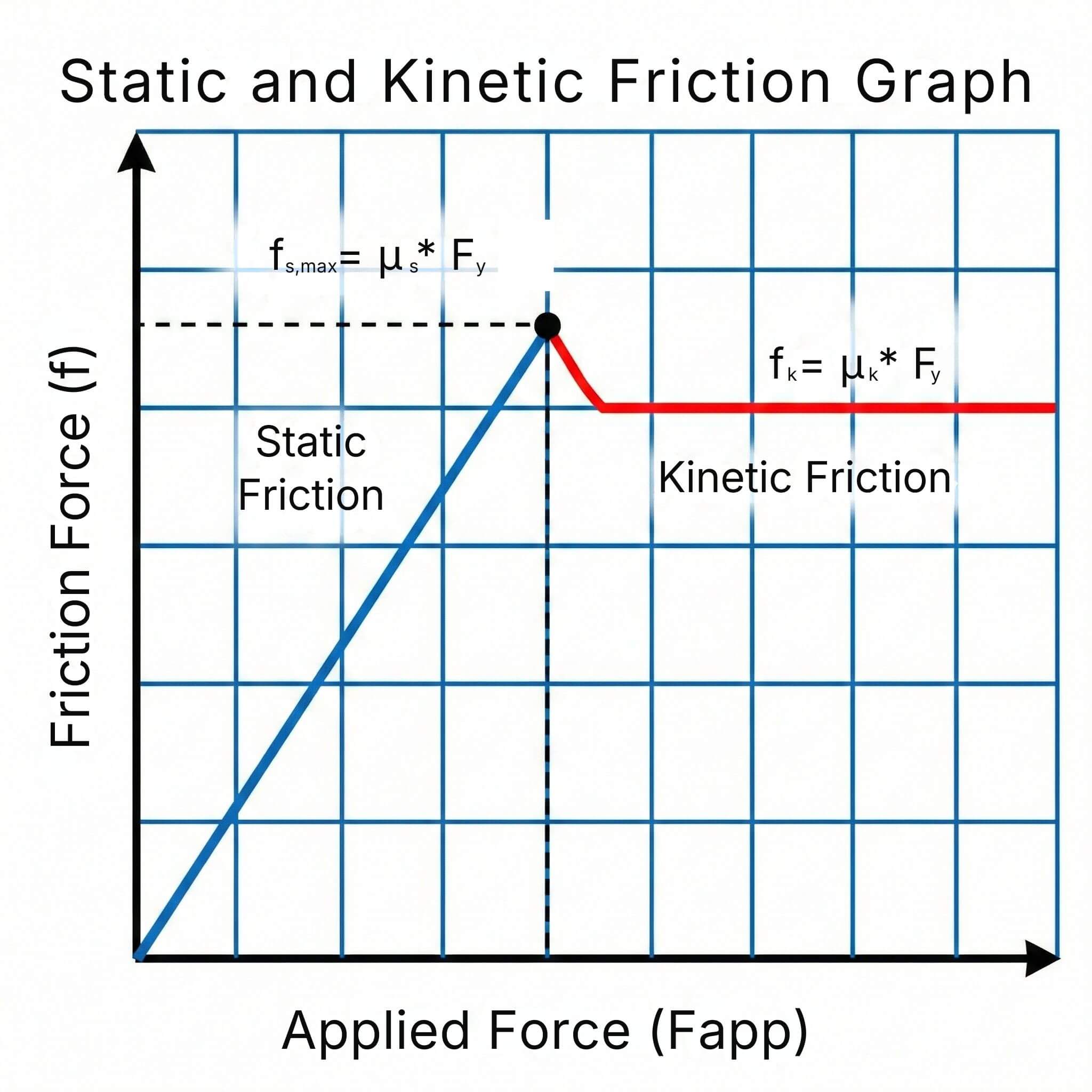
2 min read
The coefficient of friction (μ) measures the resistance to sliding between two surfaces in contact. It is a key parameter in mechanical design...

3 min read
The CBAM regulation (Carbon Border Adjustment Mechanism) represents one of the key pillars of the European strategy for industrial decarbonisation...

3 min read
Nylon is one of the most widely used plastic materials in the world.Thanks to its combination of strength, light weight, flexibility and durability,...